Trace Metals
The chemist is responsible for the analysis of priority pollutant trace metals by a specialized technique called ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry). Priority pollutant metals include antimony, arsenic, beryllium, cadmium, chromium, copper, mercury, molybdenum, nickel, lead, selenium, silver, thallium, and zinc.
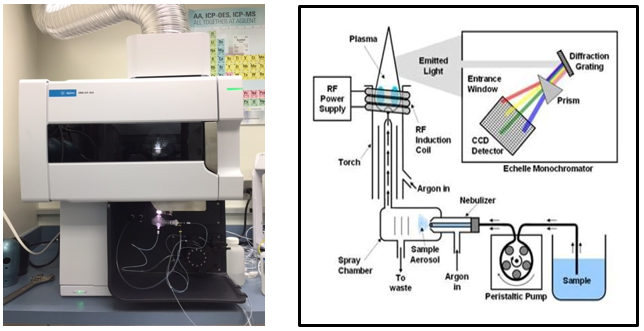
The ICP-OES is used to detect all of the metals above, with the exception of mercury (see below). A high energy plasma is created by supplying argon gas to a torch coil that excites the atoms. A sample volume is introduced into the nebulizer through an autosampler via a peristaltic pump. The sample aerosol is sent through a tube inside the torch, where the plasma energy interacts with the sample. The elements are excited by the plasma and when the excited atoms return to a low energy position, emission rays that correspond to the photon wavelength are released. The element type is determined by the position of the photon rays in the emission spectrum and the amount of each element is measured by the ray’s intensity.
Mercury
Mercury is analyzed by Cold Vapor Atomic Absorption Spectrometry (CVAAS). Sample is pumped into the mercury analyzer via a peristaltic pump and mixed with a reductant that reduces mercury to a gas. Argon gas is introduced into the liquid/gas separator to move the mercury gas into the drying and optical section where the amount of mercury is measured by its absorbency via an Atomic Absorption (AA) detector.